Abstract
Introduction: The monoclonal antibody daratumumab showed high response rates and a good safety profile in multiple myeloma and is being evaluated in clinical trials in AL amyloidosis. Light chain deposition disease (LCDD) is a rare monoclonal gammopathy of renal significance. Treatment directed against the underlying plasma cell clone can prevent renal progression. Bortezomib is commonly used upfront in these patients and daratumumab may represent a powerful novel option.
Methods: We report the outcome of six patients with refractory light chain deposition disease (LCDD) treated with daratumumab at the Amyloidosis Research and Treatment Center of Pavia. All patients gave written informed consent. Hematologic response was assessed according to the International Society of Amyloidosis criteria.
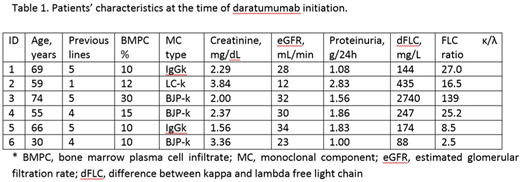
Results: Six patients (5 males and 1 female) received daratumumab intravenously at 16 mg/kg weekly for 8 weeks, followed by every other week infusions for 8 doses and then monthly infusions. Patients' clinical characteristics are reported in Table 1. All patients received daratumumab single agent except one who was treated with daratumumb and bortezomib combination (this patient received only 1 prior line of therapy). All patients were refractory to the last line of therapy. All patients received at least two months of therapy. All patients were previously treated with bortezomib, pomalidomide was used in 4 cases, lenalidomide, thalidomide and bendamustine in 2 cases each, and autologous stem cell transplant was performed in 4 subjects. The median time from LCDD diagnosis to daratumumab initiation was 8.3 years (range 8 - 147 months). Five of the 6 patients obtained hematologic response with at least a reduction of 50% of the dFLC value (partial response). Three patients obtained a very good partial response (dFLC <40 mg/L). The estimated glomerular filtration rate improved in one subject (from 30 to 45 mL/min per 1.73 m2) and in all the others remained stable. In 2 subjects treatment was temporarily discontinued due to pneumonia.
Conclusions: This is the first report of the use of daratumumab in LCDD. This antibody yielded rapid and significant hematologic responses in five of six heavily pretreated patients with this disease, preventing renal progression. Daratumumab represents a promising option for these patients and larger, international studies are warranted.
Merlini:Akcea: Consultancy; Ionis: Consultancy; Prothena: Consultancy; Millenium: Consultancy; Janssen: Consultancy; Pfizer: Consultancy. Palladini:Celgene: Other: Travel support; Janssen: Membership on an entity's Board of Directors or advisory committees; Prothena: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal